
Ammonium sulfate [(NH₄)₂ SO₄] was one of the first and most widely used nitrogen (N) fertilizers for crop production. It’s now less common but especially valuable where both N and sulfur (S) are required. Its high solubility provides versatility for a number of agricultural applications.
Production
Ammonium sulfate (sometimes abbreviated as AS or AMS) has been produced for more than 150 years. Initially, it was made from ammonia released during manufacturing coal gas (used to illuminate cities) or from coal coke used to produce steel. Today, manufacturers make it by reacting sulfuric acid with heated ammonia. To get the crystal size best suited for the application, they control the reaction conditions by screening and drying the particles until achieving the desired size. Some materials are coated with a conditioner to reduce dust and caking.
Byproducts from various industries meet most of the current demand for ammonium sulfate. For example, the nylon manufacturing process produces ammonium sulfate as a co-product. In another, certain byproducts that contain ammonia or spent sulfuric acid are commonly converted to ammonium sulfate for use in agriculture.
Although the color can range from white to beige, ammonium sulfate is consistently sold as a highly soluble crystal with excellent storage properties. As described earlier, particle size can also vary depending on the intended purpose.
Agricultural use
Growers apply ammonium sulfate primarily where they need supplemental N and S to meet the nutritional requirement of growing plants. Since ammonium sulfate contains only 21 percent N, other fertilizer sources more concentrated and economical to handle and transport often make a better choice for N-deficient fields. However, it provides an excellent source of S, which supports or drives numerous essential plant functions, including protein synthesis.
Because the N fraction is present in the ammonium form of ammonium sulfate, rice farmers frequently apply it to flooded soils, since nitrate-based fertilizers are a poor choice due to denitrification losses.
A solution containing dissolved ammonium sulfate is often added to post-emergence herbicide sprays to improve their effectiveness at weed control. This practice of increasing herbicide efficacy with ammonium sulfate works particularly well when the water supply contains significant concentrations of calcium (Ca), magnesium (Mg) or sodium (Na). A high-purity grade of ammonium sulfate often works best for this purpose to avoid plugging spray nozzles.
Management practices
After addition to soil, the ammonium sulfate rapidly dissolves into its ammonium and sulfate components. If it remains on the soil surface, the ammonium may be susceptible to gaseous loss in alkaline conditions. In these situations, agronomists advise incorporating the material into the soil as soon as possible. Other options include an ammonium sulfate application before irrigation or a predicted rainfall.
Most plants can utilize both ammonium and nitrate forms of N for growth. In warm soils, microbes will rapidly begin to convert ammonium to nitrate in the process of nitrification [2 NH₄⁺ + 3O₂ → 2NO₃⁻ + 2H₂O + 4H⁺]. During this microbial reaction, acidity [H⁺] is released, which will ultimately decrease soil pH after repeated use. Ammonium sulfate has an acidifying effect on soil due to the nitrification process, not from the presence of sulfate, which has a negligible effect on pH. The acid-producing potential of ammonium sulfate is greater than the same N application from ammonium nitrate, for example, since all of the N in ammonium sulfate converts to nitrate, compared with only half of the N from ammonium nitrate that converts to nitrate.
Non-agricultural uses
Food companies commonly add ammonium sulfate to bread products as a dough conditioner. It’s also a component in fire extinguisher powder and flame-proofing agents. And it serves many purposes in the chemical, wood pulp, textile and pharmaceutical industries.
Ammonium sulfate is used almost exclusively as a fertilizer material; minor amounts are used in nonfertilizer applications, including use as a cattle feed supplement, for several pharmaceutical applications, and for flameproofing, tanning, mining rare earth metals, food processing, fermentation, textile dyeing, and water treatment. In 2018, ammonium sulfate was used mainly (95% of world consumption) as a nitrogen fertilizer material and accounted for about 4.8% of the world nitrogen fertilizer market. Industrial use of ammonium sulfate accounts for only about 5% of world consumption.
World consumption of ammonium sulfate is concentrated in Southeast Asia, Central and South America, China, Western Europe, and the United States.
The following pie chart shows world consumption of ammonium sulfate:
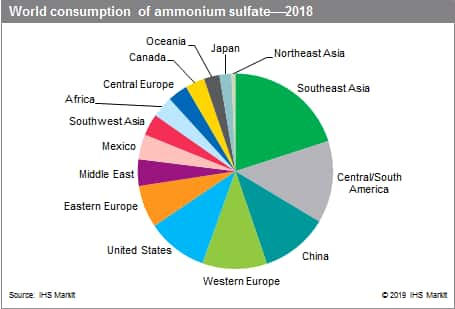
In general, the industrialized regions account for most of the world’s ammonium sulfate capacity, with Western Europe and the United States being overtaken by China in recent years. Because much of ammonium sulfate is produced involuntarily as a by+product or coproduct, the volume of production is influenced more by general industrial output levels than by fertilizer demand. As a result, the major capacity growth during 2005-18; occurred in China, and China is projected to account for the major gain during the forecast period, but at a slower rate.
Ammonium sulfate has a high sulfur content in the sulfate form, making it readily absorbable by plants. It has a low pH, making it suitable for alkaline soils. As a nitrogenous fertilizer, it competes with urea, ammonium phosphates, and ammonium nitrate. Sulfur has become increasingly recognized as an essential nutrient for plant growth since it supports the synthesis of amino acids, proteins, enzymes, vitamins, and chlorophyll. It has been found to be beneficial to a variety of crops, including canola, alfalfa, corn, potatoes, rice, vegetables, and wheat.
There are no serious environmental concerns involved with the use of ammonium sulfate as a fertilizer material. Environmental concern does, however, play an important role in the ammonium sulfate industry in that a significant portion of the world’s ammonium sulfate production is the direct result of the necessity to remove SO2 from stack gases at various metal smelting and refining operations in order to conform to government regulations on SO2 emissions. A large potential source of additional by+product ammonium sulfate production is SO2 recovery from coal+fired electrical generating stations.
In China, with the extremely rapid development of the caprolactam industry, the volume of by+product ammonium sulfate has increased constantly. Furthermore, encouraged by environmental protection policies, power plant desulfurization has also produced a rapidly increasing volume of ammonium sulfate. The increased amount went primarily to exports. Chinese exports of ammonium sulfate accounted for over two+thirds of production in China in 2018, corresponding to a strong average annual growth rate between 2013 and 2018.
The majority of ammonium sulfate produced in the United States is generated as a by+product. The US market is mature and is expected to grow at around slighltly during the next five years. In February 2017, the US International Trade Commission .USITC/ determined that the United States was materially injured by reason of imports of ammonium sulfate from China, which was sold in the United States at less than fair value and subsidized. Affected by this, imports of AS from China declined from 33,528 metric tons in 2015 to nearly zero in 2017, 2018, and the first half of 2019.
Apparent world consumption of ammonium sulfate increased steadily during 2005-18, and is forecast to continue increasing at a similar rate during 2018-23. During 2005-18, the fastest growing regions were Eastern Europe, Africa, and China. In 2018, three regions-Southeast Asia, Central and South America, and China-accounted for nearly half of total world consumption. Although the total nitrogen fertilizer market grew substantially between 2005 and 2018, and further growth is forecast by 2023, the bulk of world demand for nitrogen fertilizer is being filled by urea, another solid fertilizer material with a higher nitrogen content.