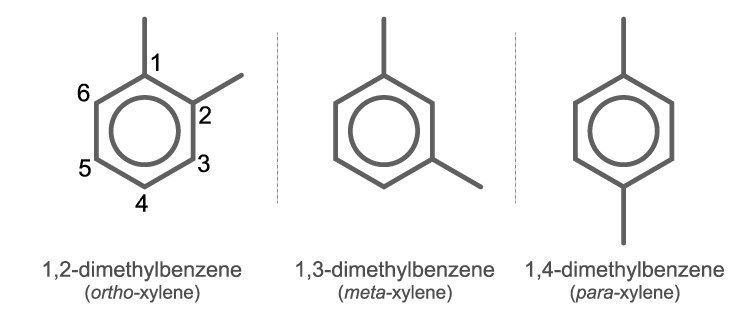
Xylene is an organic chemical compound. It is also known as dimethylbenzene or Xylol. It is one of the three isomers of dimethyl benzene. It consists of a central benzene ring attached with two methyl groups as substituents.
In the year 1850, Xylol was first isolated by a French chemist Auguste Cahours. Xylol is a colorless, clear, flammable liquid. It is produced by catalytic reforming as well as by coal carbonization. It also occurs naturally in crude oil, aircraft fuels, and gasoline. The mixture of xylene is slightly greasy and has a sweet smell, usually encountered as a solvent.
Commercially obtained Xylene is a colorless, flammable, non-viscous, toxic liquid. It does not dissolve in water but is miscible with various organic liquids. At an industrial level xylenes are synthesized by the process of methylation of benzene and toluene. The obtained Xylol contains 40 to 65% of m-xylene and approximately 20% of o-xylene, 20% of p-xylene and 20% of ethylbenzene.
Properties of Xylene (C8H10)
| Xylene | C8H10 |
| Molecular Weight | 106.16 g/mol |
| Density | 0.864 g/mL |
| Boiling Point | 138.5 °C |
| Melting Point | −47.4 °C |
Structure Of Xylene (C8H10)
Uses Of Xylene (C8H10)
- Xylene is used as a raw material for the manufacture of fibres, dyes, and films.
- Used in laboratories to cool reaction vessels.
- It is used as a clearing agent.
- It is used to thin the thick varnishes and paints.
- It was used as a tear gas agent in World War I.
Effects on Health
Inhaling xylene vapor leads to depression of the central nervous system, resulting in symptoms such as headache, vomiting, nausea and dizziness. It also leads to hearing disorders. When it comes in contact with the skin it causes irritation.